A globally competitive anticancer active agent developed in ELTE laboratory
Tumour diseases are among the leading causes of death worldwide. Since the 1990s, the rate of cancer patients has dramatically increased. In 2022, the total number of deaths caused by cancer was already almost a million and a half in the EU member states and the United Kingdom. The treatment of cancerous lesions, at the same time, represents a serious challenge. In traditional chemotherapy treatments, active agents are used as pharmaceutical ingredients to destroy the majority of tumour cells. However, they also cause severe damage to healthy cells, while leading to numerous side effects. The reoccurrence of tumours along with the appearance of metastases, as well as the fact that patients often develop resistance to chemotherapy agents also poses a serious problem.
In recent years, the so-called targeted oncological therapeutic procedures have gained extraordinary importance. In these, the agent is used to prevent the growth of tumour cells at the molecular level by inhibiting the uncontrolled multiplication of cancer cells, which malfunction in the regulation of the cell cycle. Such treatments are aimed at specific molecular targets within the cell, such as proteins. Drug molecules registered in recent years with much more favourable properties than first-generation chemotherapy agents include the small-molecule potential anticancer imipridones developed in the United States. These are aimed at the mitochondrial caseinolytic protease P (ClpP). Their most widely known representatives include the ONC-201 and ONC-212 (Fig. 1).
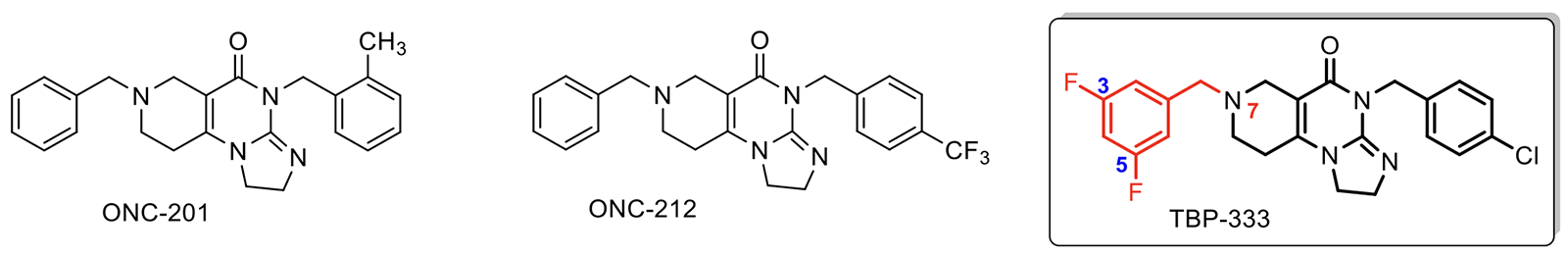
Fig. 1. The molecular structure of the ONC-201 and ONC-212 imipridones developed in the United States, already tested in pre-clinical and clinical trials, and the significantly more effective TBP-333 imipridone molecule developed in the laboratory of the ELTE Department of Organic Chemistry.
At the ELTE Faculty of Science, research into the development of new anticancer agents has been carried out for a long time funded by the Synthesis+ Excellence Programme. As a result of the innovation efforts, researchers at the ELTE Department of Organic Chemistry have so far synthesised over 140 novel imipridone-derived compounds with varied substitution patterns. Among these, the two fluorine- and one chlorine-substituted derivatives, labelled with the code name TBP-333,
have proved to be the most effective potential anti-cancer agent candidate to date
(Fig. 1). Comparative tests have revealed that the new Hungarian active agent is significantly more effective than ONC-212, which is considered to be the most promising active agent candidate by American developers. (The latter is about to enter human clinical trials involving pancreatic carcinoma and leukemia patients.)
The outstanding importance of the innovation at the international level is confirmed by the fact that TBP-333 is considerably less toxic to the body than to PANC-1 pancreatic carcinoma cells, which show a high degree of resistance to chemotherapy treatments. Consequently,
while having reduced adverse side effects, it exerts an anti-tumour effect even at doses several orders of magnitude smaller,
compared to ONC-212, being about 4000 times more potent than the latter. According to the measurement data, this means that the TBP-333 compound is able to destroy 50% of PANC-1 pancreatic carcinoma cells at a dose approximately 4000 times lower than ONC-212. The extraordinary efficacy of the active agent candidate has been confirmed by numerous other trials on human cancer cell lines. Additionally, it has been conclusively proven by animal experiments on hormone-independent aggressive breast cancer models.
The ELTE research team headed by Antal Csámpai realised through their decade-long persistent experimentation that the 3,5-difluorobenzyl group built in the imipridone core structure at position 7 – as indicated on the TBP-333 compound (Fig. 1) – is fundamental for increasing the anticancer effect drastically. This structure-effect relationship recognised by them represents the “know-how” forming the basis of their patent application.
The extraordinary competitiveness of the TBP-333 compound on the international scene is repeatedly confirmed by scientific results and control tests. The Toronto research group led by Professor Walid Houry – maintaining close research and innovation relationship with American competitors and also initiating international cooperation with Antal Csámpai and his group after the publication of the patent material – has confirmed with X-ray diffraction that the 3,5-difluorobenzyl group of the TBP-333 compound binds with exceptional complementarity to the hydrophobic pocket of the so-called “H-region” of the ClpP protease subunit located in the mitochondria generating the cell’s energy supply (Fig. 2). This binding leads to changes in the spatial structure that “expands” the heptameter channel, as a result of which the proteins generating the cell’s energy supply system are degraded. This process ultimately leads to the apoptosis of the cancerous cell.
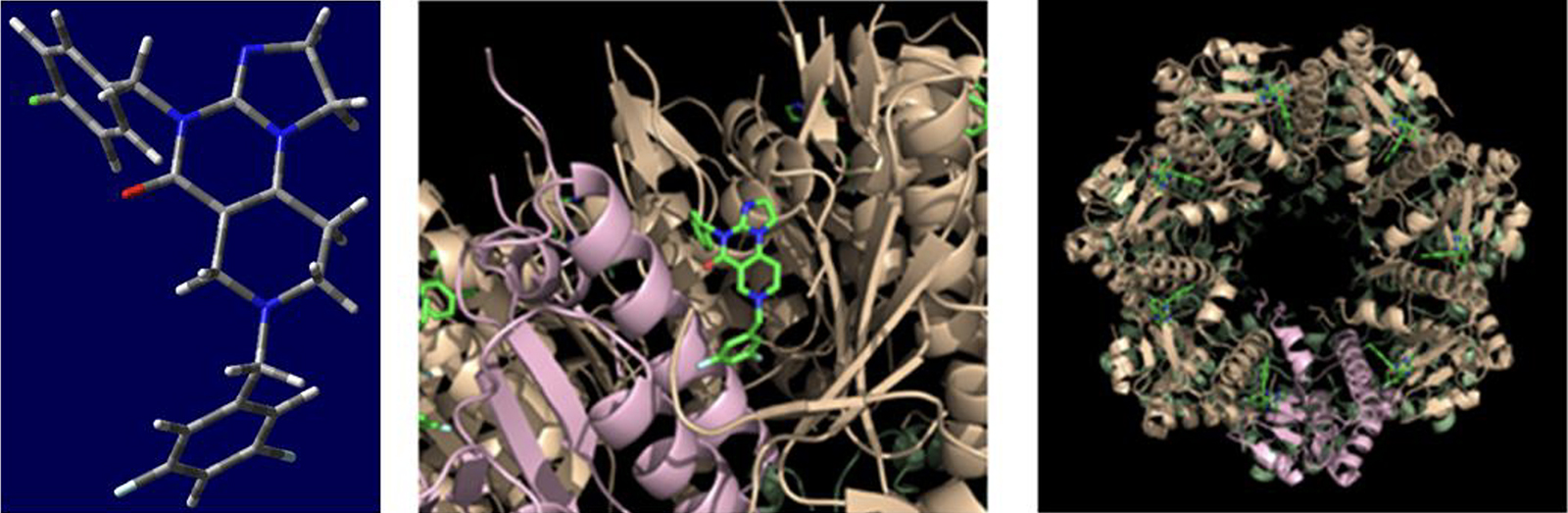
Fig. 2. on the left: the spatial structure model of the TBP-333 compound capable of binding to the target ClpP protease; in the middle: an enlarged detail of the complex formed by the TBP-333 molecules and ClpP; on the right: the ClpP forming a heptametric complex with the seven TBP-333 molecules bound to it.
It has also been demonstrated that the effect of TBP-333 on certain tumour cells characterised by a high death rate, including the hormone-independent MDA-MB-231 aggressive breast cancer cells, far surpasses the activity of the TR-107 compound (Fig. 3), which is the most potent representative of the third-generation ClpP activators developed by American scientists. Moreover, the variant of TR-107 compound containing a 3-cyanobenzyl group and modified with a 3,5-difluorobenzyl group synthesised by ELTE scientists (Fig. 3) was considerably more potent against the above-mentioned cells than the TR-107 compound, but did not reach the efficacy of the TBP-333 compound.
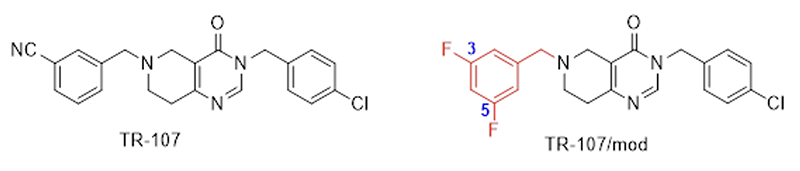
Fig. 3. The third-generation ClpP activator molecule (TR-107) developed in the United States and its more potent variant – modified with a 3,5-difluorobenzyl group that ensures exceptionally strong ClpP binding and thus activating the protease – synthesised by ELTE researchers.
Based on the above information, the development of the TBP-333 active agent candidate for the purpose of clinical therapeutic application is predicted to be a far-reaching and outstanding success. This primarily requires extensive biological research (cellular, functional, and toxicity testing) as well as further experiments on animals. The use of methods specialised for the identification and structural analysis of chemical and biological samples with complex compositions, such as nuclear magnetic resonance techniques, is also indispensable for the trials.

